Optical Activity
If the words levo and dextro ring a bell, you probably learned this at some point. Optical activity is the way chiral molecules bend polarized light a certain way. Polarized light is light in one plane. In the image below, the light is all polarized to be only vertical.
 |
| An example of polarization |
 Another important detail of optical activity is what I mentioned earlier: dextro and levorotation.If a molecule is dextrorotary, it turns plane polarized light clockwise. If it is levorotary, it turns it counterclockwise. Two enantiomers will turn light exactly the same amount in opposite directions. So if you have a racemic mixture (see Enantiomeric Excess for more detail), the light won't be turned at all!
Another important detail of optical activity is what I mentioned earlier: dextro and levorotation.If a molecule is dextrorotary, it turns plane polarized light clockwise. If it is levorotary, it turns it counterclockwise. Two enantiomers will turn light exactly the same amount in opposite directions. So if you have a racemic mixture (see Enantiomeric Excess for more detail), the light won't be turned at all!
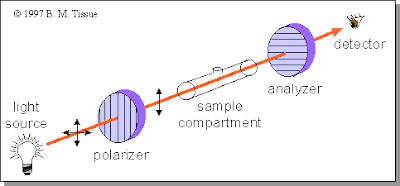
The polarization and detection of the light and how the molecules affect it are done in a polarimeter. This device shoots polarized light through a compartment holding a sample of the chemical, and analyzes how much light passes through another lens. The second lens, the analyzer, is turned exactly 90° to the polarizer, causing it to filter out light that is vertical. Then, a detector on the end tells how much light passed through.
No comments:
Post a Comment